 Clark's Nutcracker, High Sierra Photo©CLCase |
|
A standard curve is a graph relating a measured quantity (e.g., radioactivity, fluorescence, or optical density) to concentration of the substance of interest in "known" samples. You prepare and assay "known" samples containing the substance in amounts chosen to span the range of concentrations that you expect to find in the "unknown" samples. You then draw the standard curve by plotting assayed quantity (on the Y axis) vs. concentration (on the X axis). Such a curve can be used to determine concentrations of the substance in "unknown" samples
Preparation of a standard curve-to get a suspension containing a known
number of bacteria:
1. Obtain a 24 - 48-hr culture of bacteria.
2. Standardize a spectrophotometer with sterile nutrient broth.
3. Determine the abs. of the culture.
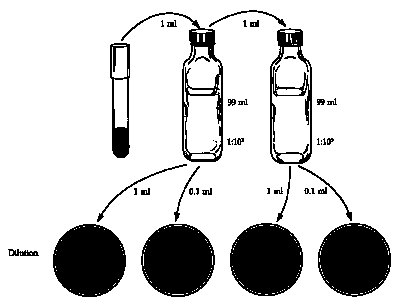
4. Transfer 1.0 ml of the culture to a bottle containing 99 ml of nutrient broth to make a 1:100 dilution. Determine the abs. of the 1:100 dilution.
5. Transfer 1.0 ml of the 1:100 dilution to another 99-ml bottle of nutrient broth to make a 1:104 dilution. Determine the abs. of the 1:104 dilution.
6. Transfer 1.0 ml of the 1:104 dilution to another 99-ml bottle of nutrient broth to make a 1:106 dilution. Determine the abs. of the 1:106 dilution.
7. Complete the following table:
|
Abs. (Y axis) |
1:100 |
|
1:104 |
|
1:106 |
|
A. Use the original undiluted culture.
1. Using a sterile 1-ml pipette, aspecially transfer 1.0 ml of the bacteria into a 99-ml dilution blank, label the tube "1:102" and discard the pipette.
2. Using a different sterile 1-ml pipette, transfer 1.0 ml of the 1:100 dilution to a 99-ml dilution blank, label the tube "1:104.
3. Mark the bottoms of 4 sterile nutrient agar plates with the dilution: 1:105, 1:104, 1:103, 1:102.
4. Using a sterile 1-ml pipette, aspetically transfer 0.1 ml of the 1:104 to the 10-5 plate and 1.0 ml to the 1:104 plate.
5. Aseptically transfer 0.1 ml of the 1:102 dilution to the 103 plate and 1.0 ml to the 102 plate.
6. Sterilize a spreading rod by dipping it in alcohol and burning off the alcohol. Spread the bacteria over the surface of the agar using the sterile spreading rod. Start with the highest dilution. Why?

7. Incubate the plates inverted at 37°C for 24-48 hr.
8. Select the plate with between 25-250 colonies. Count the colonies and determine the number colony forming units per milliliter (cfu/ml) in the original sample. See actual plates: Sample 1 | Sample 2
B. Repeat steps 1-8 with each dilution prepared above to complete the following table:
|
Abs. |
cfu/ml (Y axis) |
1:100 |
|
|
1:104 |
|
|
1:106 |
|
|
Plot abs. (Y axis) and cfu/ml (X axis).
How can you quickly use this graph to prepare a culture containing 1000 cells/ml from a turbid 24-hr culture of bacteria?