|
Christine L.Case |
You will be provided a concentrated solution of your test chemical (C1). You will need to search scientific literature to find the range of concentrations of your test chemicals that are found in the environment and what range of concentrations are reasonable to test. To dilute your test chemical to a working concentration (C2):
C1 x V1 = C2 x V2
Serial dilutions are used to test the effects of a varying concentrations.For convenience, dilutions are made in multiples of two or ten.
A single dilution is calculated as follows:
Dilution = ![]()
For example, the dilution of 1 ml into 9 ml =
![]() , which is
, which is
![]() = 1:10 =
10-1
= 1:10 =
10-1
This same formula applies for all dilutions, regardless of the volumes. A dilution of 1 ml into 1 ml of diluent equals
![]() , which is
, which is
![]() = 1:2
= 1:2
A dilution of 0.1 ml into 0.9 ml of diluent (water) equals:
![]() , which is
, which is
![]() =
= ![]() = 1:10 = 10-1
= 1:10 = 10-1
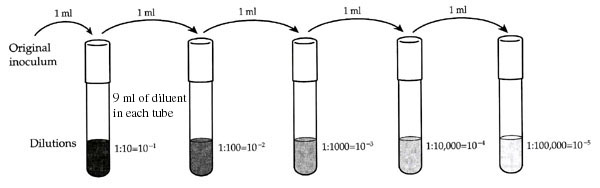
Better accuracy is obtained with very large dilutions if the total dilution is made out of a series of smaller dilutions rather than one large dilutions. This series is called a serial dilution, and the total dilution is the product of each dilution in the series. For example, if 1 ml is dilution with 9 ml, and then 1 ml of that dilution is put into a second 9 ml diluent, the final dilution will be
![]() X
X ![]() =
= ![]() = 1:100
= 10-2
= 1:100
= 10-2
Try some practice problems