Red-eyed tree frog Photo©CLCase |
Enzyme Kinetics(BIOL 230, Lab Experiment 11)
|
The purpose of this web site is to provide
a guide to analyzing enzyme kinetics.
Sample Vmax and Km calculations
Protease inhibitors
Sample lab data & graphs
Sample Vmax and Km calculations | Go to Lockey Program in Flash | in HTML
A Run the experiment at different
concentrations of acetylcholine (Ach). Here are some sample data (these
are not actual data from the experiment):
Time (min) |
Amount of HAc produced |
|||
|
at 0.5 mM Ach |
at 1.0 mM Ach |
at 2.0 mM Ach |
at 3.0 mM Ach |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.1 |
0.2 |
0.2 |
0.3 |
0.3 |
0.2 |
0.4 |
0.5 |
0.5 |
0.6 |
0.3 |
0.5 |
0.7 |
0.8 |
0.8 |
0.4 |
0.5 |
0.8 |
1.1 |
1.1 |
0.5 |
0.5 |
0.9 |
1.3 |
1.4 |
0.6 |
|
1.0 |
1.5 |
1.6 |
0.7 |
|
1.0 |
1.7 |
1.9 |
0.8 |
|
|
1.9 |
2.2 |
0.9 |
|
|
2.0 |
2.4 |
1.0 |
|
|
2.0 |
2.6 |
1.1 |
|
|
2.0 |
2.8 |
1.2 |
|
|
|
2.9 |
1.3 |
|
|
|
3.0 |
1.4 |
|
|
|
3.0 |
1.5 |
|
|
|
3.0 |
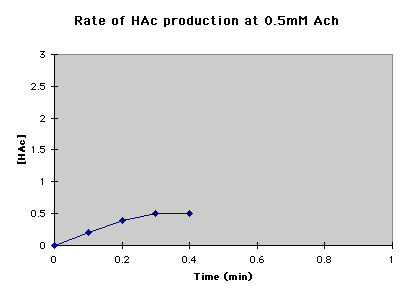
B. For each concentration, plot the acetic acid (HAc) produced versus time. |
|||||||||||||
|
|
||||||||||||
|
|
||||||||||||
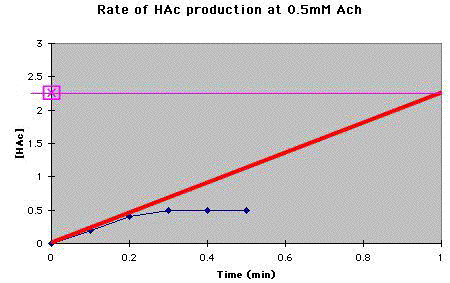
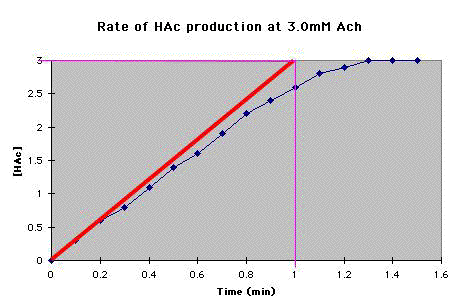
C. Draw the tangent (thick red line) to the fastest part of the reaction, out to 1 minute. To get the amount of HAc produced per minute (thin magenta line). |
|||||||||||||
|
|
||||||||||||
|
At 0.5 mM Ach, the rate of HAc production was 2.25mM/min. |
||||||||||||
|
|
||||||||||||
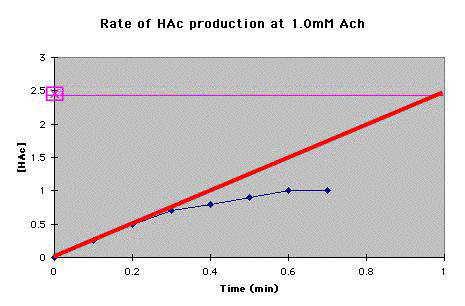
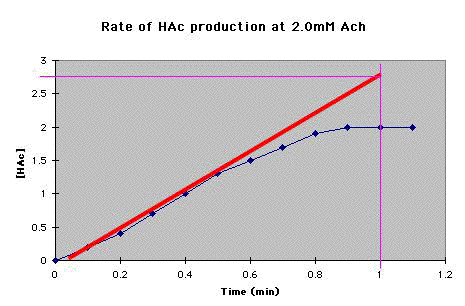
D. Continue steps A and B for each concentration of Ach for which you have data. |
|||||||||||||
|
|
||||||||||||
|
How much HAc is produced per minute? _____ (The answer is in E, below.) |
||||||||||||
|
|
||||||||||||
|
mM HAc/min = _____ (The answer is in E, below.) |
||||||||||||
|
|
||||||||||||
|
HAc/min = _____ (The answer is in E, below.) |
||||||||||||
|
|
||||||||||||
E. You have now collected the following data: |
|||||||||||||
|
|
||||||||||||
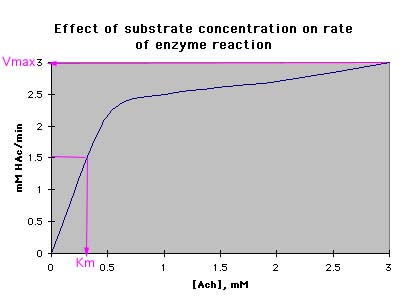
F. Plot these data to determine the Km and Vmax for this enzyme. |
|||||||||||||
|
|
||||||||||||
|
The Vmax is 3.0 mM HAc/min. The Km is 0.3 mM Ach. |
||||||||||||
G. Assume you ran this reaction with different inhibitors and got the following data: |
|||||||||||||
|
|
||||||||||||
|
A competitive inhibitor shows the max. rate at a higher concentration (Km) than normal because it binds the enzyme causing the enzyme "wastes" time bound to the inhibitor. |
||||||||||||
|
|
||||||||||||
|
A noncompetitive inhibitor gives a slower rate (Vmax) because it binds the enzyme changing the active site of the enzyme. |
||||||||||||
|
|
||||||||||||
|
Which inhibitor (#1 or #2) is a competitive inhibitor? A noncompetitive inhibitor? |
||||||||||||
|
|||||||||||||